Protocol fotodinamic cu porfirine inovative și modulatori redox în patologia cutanată premalignă - demonstrare preclinică
PORPHYDERM
Cod proiect: PN-III-P2-2.1-PED-2021-0360
Număr contract: 637PED/2022
Program: Proiect Experimental Demonstrativ (PED)
Domeniu: 1.3 – Biotehnologii
Durata proiectului: 28.06.2022 – 27.06.2024 (24 luni)
Instituții partenere
Proiectul este implementat de un consortiu multidisciplinar care are o lungă istorie de colaborare și expertiză în dezvoltarea preclinică de porfirine noi pentru PDT, precum și în medicina și terapia redox.
- Coordonator: Institutul Național de Cercetare – Dezvoltare în Domeniul Patologiei și Științelor Biomedicale „Victor Babeș”, Director de proiect: CSI Dr. Gina MANDA
- Partener 1: Universitatea de Medicină și Farmacie “Carol Davila”, Responsabil partener P1: Prof. Rica BOSCENCU
- Partener 2: Biotehnos SA, Responsabil partener P2: CSI Dr. Laura OLARIU.
Buget
- Valoarea totală de la buget: 598.795 RON
- Valoarea totală a cofinanțării (surse proprii): 53.895 RON
- Valoarea totală a Contractului: 652.690 RON
Rezumat
Proiectul are ca obiectiv principal dezvoltarea la nivel preclinic a unui protocol personalizat de terapie fotodinamică (PDT) utilizând fotosensibilizatori porfirinici inovativi și modulatori redox, formulați inteligent pentru tratamentul leziunilor cutanate premaligne, cum ar fi cheratoza actinica (AK). Proiectul se încadrează în tematica Bioeconomie – Biotehnologie.
Aplicația medicală va fi demonstrată utilizând celule și modele de șoareci. Se va investiga atât eficacitatea terapeutică, cât și aspectele toxicologice ale noului protocol PDT. Vom începe proiectul la nivelul TLR 2 – formularea tehnologiei/conceptului de produs, bazându-ne pe un portofoliu de șase brevete și expertiza echipei în domeniul PDT și al medicinei redox. Vom avansa prin TRL 3 – validarea conceptului experimental, prin studii in vitro si ex vivo utilizând linii celulare și explanturi de piele de la șoarece. Ne așteptam să ajungem la sfârșitul proiectului la TLR 4 – validarea în laborator a tehnologiei/metodei, prin testarea în model animal a unui protocol inovativ de keratoliză prin PDT, cu aplicație în AK, care are la bază fotosensibilizatori inovatori si modulatori redox.
OBIECTIVE
Obiective principale
Proiectul are ca obiectiv principal dezvoltarea la nivel preclinic a unui protocol personalizat de terapie fotodinamică (PDT) utilizând fotosensibilizatori porfirinici inovativi și modulatori redox, formulați inteligent pentru tratamentul leziunilor cutanate premaligne, cum ar fi cheratoza actinica (AK). Proiectul se încadrează în tematica Bioeconomie – Biotehnologie.
Aplicația medicală va fi demonstrată utilizând celule și modele de șoareci. Se va investiga atât eficacitatea terapeutică, cât și aspectele toxicologice ale noului protocol PDT. Vom începe proiectul la nivelul TLR 2 – formularea tehnologiei/conceptului de produs, bazându-ne pe un portofoliu de șase brevete și expertiza echipei în domeniul PDT și al medicinei redox. Vom avansa prin TRL 3 – validarea conceptului experimental, prin studii in vitro si ex vivo utilizând linii celulare și explanturi de piele de la șoarece. Ne așteptam să ajungem la sfârșitul proiectului la TLR 4 – validarea în laborator a tehnologiei/metodei, prin testarea în model animal a unui protocol inovativ de keratoliză prin PDT, cu aplicație în AK, care are la bază fotosensibilizatori inovatori si modulatori redox.
Obiective secundare
Proiectul are ca obiectiv principal dezvoltarea la nivel preclinic a unui protocol personalizat de terapie fotodinamică (PDT) utilizând fotosensibilizatori porfirinici inovativi și modulatori redox, formulați inteligent pentru tratamentul leziunilor cutanate premaligne, cum ar fi cheratoza actinica (AK). Proiectul se încadrează în tematica Bioeconomie – Biotehnologie.
Aplicația medicală va fi demonstrată utilizând celule și modele de șoareci. Se va investiga atât eficacitatea terapeutică, cât și aspectele toxicologice ale noului protocol PDT. Vom începe proiectul la nivelul TLR 2 – formularea tehnologiei/conceptului de produs, bazându-ne pe un portofoliu de șase brevete și expertiza echipei în domeniul PDT și al medicinei redox. Vom avansa prin TRL 3 – validarea conceptului experimental, prin studii in vitro si ex vivo utilizând linii celulare și explanturi de piele de la șoarece. Ne așteptam să ajungem la sfârșitul proiectului la TLR 4 – validarea în laborator a tehnologiei/metodei, prin testarea în model animal a unui protocol inovativ de keratoliză prin PDT, cu aplicație în AK, care are la bază fotosensibilizatori inovatori si modulatori redox.
SCHEMA STUDIULUI
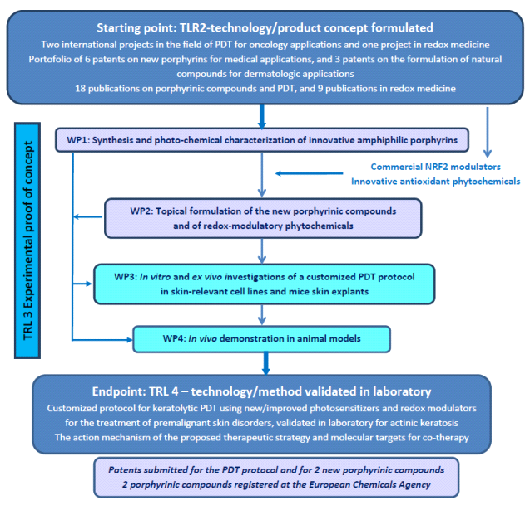
REZULTATE ESTIMATE
1) Cel puțin 2 compuși porfirinici noi pentru tratamentul cheratozei actinice (AK) cu proprietăți îmbunătățite în comparație cu fotosensibilizatorii comerciali. Sunt de așteptat îmbunătățiri în ceea ce privește amfifilicitatea, fluorescența pentru imagistica in vivo, randamentul semnificativ de oxigen singlet pentru PDT și stabilitatea bună / păstrarea proprietăților fotodinamice în formularea topică a fotosensibilizatorilor.
2) Specificații tehnice pentru toți compușii porfirinici și fitocompușii formulați, precum și pentru protocolul PDT selectat și rezultatul acestuia la șoarecii cu AK.
3) Documentația pentru cel puțin 3 cereri de brevet, depusă la Oficiul Român de Brevete și Mărci.
4) Doi compuși porfirinici înregistrați la Agenția Europeană pentru Produse Chimice.
5) Cel puțin 2 publicații în reviste cu factor de impact > 4 și cel puțin 2 comunicări la congrese relevante din domeniul dermatologiei, toxicologiei și biologiei redox (luăm în considerare că rezultatele asociate brevetului nu trebuie publicate anterior).
6) Un workshop organizat la finalul proiectului pentru prezentarea rezultatelor părților interesate (mediul academic, dermatologi și IMM-uri farmaceutice).
7) Noi servicii de cercetare privind testarea preclinică a noilor strategii PDT în dermatologie, care urmează să fie implementate la: IVB (PDT pe celule tumorale și animale mici de laborator) și la BTH (PDT in vitro pe celule relevante pentru piele, proces tehnologic pentru formularea de porfirine și modulatori redox fotochimici), care urmează să fie publicate în eERIS.
8) Dezvoltare detaliată și strategie de piață, profitând și de Acțiunea COST CA20121 care creează cadrul de colaborare cu IMM-urile farmaceutice din medicina redox și farmaceutică.
9) Pagina web și canale de media dedicate pentru promovarea cunoștințelor generate în proiect către specialiști, industria farmaceutică și publicul larg.
REZUMATUL ETAPEI I/2022
Sinteza și caracterizarea preliminară a noilor compuși porfirinici
Statisticile recente evidențiază cancerul ca principală cauză de deces la nivel mondial, prevăzând o creștere a numărului de cazuri față de anul 2020 cu 47% până în anul 2040 [1,2]. Din aceste considerente dezvoltarea de strategii inovative privind diseminarea măsurilor de preventie și furnizarea de substanțe active cu potențial antitumoral sunt esențiale pentru combaterea cancerului la nivel mondial. Leziunile non-melanom (keratoze actinice, carcinom spinocelular și bazocelular, limfom cutanat, sarcom Kaposi, angiosarcom) sunt printre cele mai frecvente forme de cancer. Dezvoltarea unui cancer non-melanom este determinată de factori genetici, istoric de infecție HPV, istoric de afecțiuni cutanate inflamatorii cronice, expunere la substanțe toxice, la soare, imunosupresie, fototipurile cutanate I și II, PUVA ca terapie anterioară [3, 4]. Keratozele actinice sunt descrise in literatura sub forma de ,,carcinom in situ” (din cauza keratinocitelor displazice asemănătoare cu carcinomul cu celule scuamoase) sau ca leziune premalignă [5]. Abordarea terapeutica în cazul keratozei actinice trebuie să ținteasca scăderea riscului de malignizare și un diagnostic precoce al formațiunilor maligne care pot apărea într-un camp de keratoză actinică. Porfirinele, prin profilul lor structural si spectral, au potential în identificarea și terapia manifestărilor cutanate de tip malign [6]. În plus, avantajul major al acestor tipuri structurale asemănătoare hemului este selectivitatea pentru celule tumorale și versatilitatea lor, posibilitatea modelării structurale prin atașare de substituenți cu diferite grade de polaritate, pentru obtinerea unui raport hidrofil/lipofil optim pentru internalizarea celulară.
In acest context, obiectivul principal al proiectului este de a elabora un protocol pentru terapia fotodinamica (PDT) a keratozei actinice, utilizand noi derivati porfiriniici. Activitatile din cadrul proiectului includ evaluarea potentialului de marker si agent antitumoral al unor compusi porfirinici cu arhitectura moleculara asimetrica si profil spectral adecvat. O primă etapă a proiectului prevede obținerea de noi structuri porfirinice, prin tehnici ecologice de sinteză care respectăa cerințele actuale din domeniul sintezei medicamentului. S-a urmărit obținerea unui set de date asociate procedurilor de sinteză și parametrilor care definesc profilul structural al noilor fotosensibilizatori.
In cadrul Activitatii I.1 realizată de UMF au fost obținuti următorii compuși porfirinici:
- cu structuri asimetrice:
- 5-(2,4-dihidroxifenil)-10,15,20-tris-(4-acetoxi-3-metoxifenil) porfirina (2)
- 5-(2-hydroxi-3-metoxifenil)-10,15,20-tris-(4-carboximetilfenil) porfirina (P 5.2)
- cu structuri simetrice (referință pentru derivații asimetrici în studiile in vitro)
- 5,10,15,20-meso-tetrakis-(4-acetoxi-3-metoxifenil) porfirina (P4.1º1)
- 5, 10,15,20-tris-(4-carboximetilfenil) porfirina (P 5.1)
Evaluarea structurală și spectrală a compușilor sintetizați s-a realizat în Activitatea I.1 prin analiza RMN, FTIR, UV-Vis și fluorescență. Corelarea datelor obținute a confirmat structurile compușilor porfirinici sintetizați în această etapă. Concluzionând, studiile preliminare au condus la obtinerea, cu randamente bune, a 4 compusi porfirinici prin abordarea unei metode de sinteza moderne si ecologice.
În Activitatea I.2 au fost analizate 3 tipuri de formulări pentru aplicații topice ale compușilor porfirinici și modulatorilor, întro primă etapă fiind selecționată și detaliată metoda în gel.
Studiul in vitro realizat în Activitatea I.3 a arătat că toți compușii porfirinici analizați, evaluați in vitro pe celule normale și tumorale specifice pielii (keratinocite umane normale HaCaT, fibroblșsti umani dermici HS27 și celule de mealnom de șoarece B16F10) au avut o bună biocompatibilitate cu celulele normale și tumorale de piele. Se remarcă totuși următoarele aspecte: 1) compusul P5.2 poate inhiba multiplicarea keratinocitelor, ceea ce are relevanță terapeutică în cazul keratozei actinice caracterizată prin hiper-proliferarea acestor celule, dacă se evită fotosensibilizarea temporară a pielii; b) compușii P2.1 și P5.2, dar nu și derivatul porfirinic P4.2, determină creșterea eliberării LDH de către fibroblaștii dermici, posibil datorită unor efecte nedorite care perturbă integritatea membranară a fibroblaștilor dermici.
Toți compușii porfirinici analizați s-au incorporat în celulele normale și tumorale de piele investigate, este adevărat cu intensități diferite, dovedind astfel proprietăți de marcator fluorescent. Compușii porfirinici P2.1 și P4.2 pot fi considerați candidați pentru imagistică de fluorescență în dermatologie, și pentru PDT în dermatologia oncologică.
Pentru vizibilitatea proiectului și a realizărilor sale, în Activitatea I.4 a fost realizată pagina web a proiectului, care se găseste la adresa
În Activitatea I.5, coordonată de UMF a fost elaborată cererea de brevet de invenție cu titlul ,,Compus porfirinic cu potential de marcator fluorescent in dermato-oncologie”, la care au participat toți partenerii. Documentatia tehnică a cererii de brevet revendică elemente de noutate corespunzatoare obținerii, evaluării spectrale și biologice a compusului 5-(2-hydroxi-3-metoxifenil)-10,15,20-tris-(4-carboximetilfenil) porfirina (P5.2). Cererea de brevet de invenție a fost înregistrată la OSIM.
Obiectivul și activitățile etapei I au fost realizate 100%.
REZUMATUL ETAPEI II/2023
Studiu preclinic intermediar
Activitățile etapei II
Activitate | Partener |
Activitatea II.1 Sinteza si caracterizarea unor compusi porfirinici pentru studiile preclinice | UMF (P1) |
Activitatea II.2 Obtinerea, formularea si caracterizarea unor fitocompusi | BTH (P2) |
Activitatea II.3 Studiu biologic in vitro | IVB (CO) |
Activitatea II.4 Formularea compusilor porfirinici si a modulatorilor redox pentru aplicatie topica | BTH (P2) |
Activitatea II.5 Studiu biologic ex vivo pe explanturi de piele | BTH (P2) |
Activitatea II.6 Realizarea si caracterizarea modelelor animale de cheratoza actinica | IVB (CO) |
Activitatea II.7 Studiu preliminar in vivo in model animal | IVB (CO) |
Activitatea II.8 Evaluarea homeostaziei pielii in model animal de cheratoza actinica cu diverse tratamente | BTH (P2) |
Activitatea II.9 Diseminarea rezultatelor: actualizarea paginii web, articole, comunicări, parteneriat internațional. | IVB (CO) UMF (P1) |
Rezultatele etapei II
Rezultate estimate | Rezultate realizate |
2 compusi porfirinici selectati si specificatii tehnice intermediare | 3 compusi porfirinici cu specificatii tehnice: P2.1, P2.2 și P4.2 (Anexa 1) |
2 fitocompusi cu specificatiile lor tehnice | 2 fitocompusi cu specificatii tehnice (Activitatea II.2 si Anexa 2) |
1 raport final de studiu biologic in vitro | Raport de studiu preliminar in vitro (descris la Activitatea II.3. Rezultatele sunt prezentate in articolul publicat in revista ISI (Anexa 3 si Anexa 9-articol submis pentru publicare) |
1 raport de studiu intermediar pe explanturi de piele | • Metoda de obtinere si testare explanturilor de piele in vederea studiilor de difuziune verticala a preparatelor topice si transdermice (vezi si Anexa 5.1); • Metoda HPLC pentru compusi porfirinici; • Protocol de realizare a PDT pe explanturi de piele (Anexa 5.2). |
2 compusi porfirinici și 2 fitocompusi formulati pentru aplicatie topica | Variante de geluri pentru formularea topica a fotosensibilizatorilor si a fitocompusilor pentru PDT la nivelul pielii (Activitatea II.4, Anexa 4) |
1 protocol de PDT ex vivo pe explanturi de piele | Protocol de PDT ex vivo pe explanturi de piele (Anexa 5) |
2 modele animale de cheratoza actinica | 2 modele animale caracterizate (expunere repetata la UVB, tumori transplantabile de celule de carcinom uman de piele cu celule scuamoase in soareci nuzi imunosupresati); Un al treilea model este in curs de realizare (carcinogeneza chimic indusa in soareci FAV). Vezi Activitatea II.6 si Anexa 6. |
1 raport de studiu preliminar in vivo | Raport de studiu preliminar in vivo pe soareci C57BL/6 expusi repetitiv la doze crescatoare de UVB si tratati in vivo cu (Activitatea II.7). |
Panel de gene tinta ale NRF2 | Panel de gene tinta ale NRF2, relevant pentru terapia fotodinamica (Anexa 7.1) |
1 protocol de terapie combinată PDT și modulare redox in model preclinic | 1 protocol de terapie combinată PDT și modulare redox in model preclinic (Anexa 7.2) |
1 procedură de investigare a pielii in vivo | Procedura de investigare a pielii in vivo (Activitatea II.8 si Anexa 8) |
1 pagina web actualizată | PORPHYDERM – National Institute of Pathology Victor Babeş – Bucharest (ivb.ro) |
2 comunicari stiintifice
| · 1 comunicare stiintifica la Conferinta Stiintifica de toamna a AOSR 2023 cu titlul ”Știința pentru o societate sănătoasă”, organizata in perioada la 21-23.09.2023 Universitatea OVIDIUS din Constanța; · 1 prezentare orala in domeniul factorului de transcriptie NRF2 in cancer la Cursul „NRF2 in Noncommunicable Diseases: from Bench to Bedside”, organizat in cadrul Actiunii COST CA20121 in perioada 26-30.06.2023 la smolenice Castle, Slovacia. |
2 articole stiintifice publicate | · 1 articol publicat in anul 2023 la revista Molecules (ISI, factor de impact 2023 4.927); · 1 articol depus pentru publicare in revista Pharmaceuticals (ISI, factor de impact 5.215). |
2 cereri de brevet depuse la OSIM | · 1 cerere de brevet a fost depusă la OSIM cu nr. de înregistrare A/00775/28.11.2022 și cu titlul ,,Compus porfirinic cu potential de marcator fluorescent in dermato-oncologie” (depunere raportata în avans în etapa I) · Pentru unul dintre compusii testati in cadul acetsei etape a fost acordat berevetul No. 132752 B1, publicat in RO-BOPI, 11 din 29.11.2023, cu titlul „ Porphyrin derivative for theranostic use”, autori: Rica Boscencu, Gina Manda, Radu Petre Socoteanu, Mihail Eugen Hinescu, Ionela Victoria Neagoe, Laura Olariu, Brandusa Dumitriu. |
1 cerere de brevet in curs de elaborare | 1 cerere de brevet este in curs de elaborare privind formularea fotosensibilizatorilor selectionati in etapele 1 si 2 ale proiectului pentru aplicatie topica in PDT. |
2 protocoale de colaborare cu experti din strainatate | 3 protocoale de colaborare cu experti din strainatate |
REZUMATUL ETAPEI III/2024
Studiu de validare a rezultatelor obtinute
Activitatile etapei III
Activitate | Partener |
Activitatea III.1 Sinteza si caracterizarea compusilor porfirinici selectati pentru studiul de validare |
UMF (P1) |
Activitatea III.2 Obtinerea, formularea si caracterizarea compusului porfirinic si a fitocompusului selectionat pentru studiul de validare |
BTH (P2) UMF (P1) |
Activitatea III.3 Studiu biologic de validare ex vivo a terapiei combinate PDT si modulare redox pe explanturi de piele de la soareci cu cheratoza actinica |
BTH (P2) IVB (CO) |
Activitatea III.4 Studiu biologic de validare in vivo a terapiei combinate PDT si modulare redox la soareci cu cheratoza actinica |
IVB (CO) |
Activitatea III.5 Diseminarea rezultatelor | IVB (CO) UMF (P1) |
Activitatea III.5.1 Actualizarea paginii web, realizarea unui protocol de transfer tehnologic intre parteneri, organizarea unui workshop demonstrativ |
IVB (CO) |
Activitatea III.5.2 Elaborarea documentatiei tehnice finale a studiului preclinic si diseminarea acesteia |
UMF (P1) |
Rezultatele etapei III
Rezultate estimate | Rezultate realizate |
1 compus porfirinic validat si specificatia sa tehnica finala | Compusul porfirinic P2.2 validat in vitro si in model animal de carcinomatoza chimic indusa Compusii porfirinici P2.1 si P4.2 validati in vitro Specificatiile tehnice ale produsilor P2.1, P2.2 si P4.2 |
1 compus porfirinic formulat | 2 compusi porfirinici (P2.1 si P2.2) formulati in gel pentru aplicatie topica (chitosan si hidroxipropilmethilceluloza) 2 documentatii de brevet pentru gelurile cu porfirina |
1 fitocompus formulat si specificatia sa tehnica | 1 fitocompus formulat si specificatia tehnica pentru produs |
1 raport de studiu de validare ex vivo | 1 raport de studiu in vivo in model animal de soareci expusi cronic la UVB Baza de date cu parametri ai pielii determinati prin metode neinvazive la soareci expusi cronic la UVB si tratati cu PDT sau cu modulatori redox |
1 protocol de investigare ex vivo a pielii | Protocol de investigare ex vivo a pielii |
1 studiu biologic de validare in vivo | Validarea in vivo a compusului porfirinic P2.2 in model animal de cheratoza actinica (carcinomatoza indusa chimic) si in model animal de expunere cronica la UVB |
1 portofoliu de metode experimentale | · Sinteza compusilor porfirinici · Caracterizarea fizico-chimica a compusilor porfirinici · Caracterizarea fizico-chimica si farmaceutica a gelurilor cu porfirine pentru aplicatie topica · PDT in vitro si in vivo · Procedura de investigare neinvaziva a pielii la soarece |
1 pagina web actualizata | PORPHYDERM – National Institute of Pathology Victor Babeş – Bucharest (ivb.ro) |
1 protocol de transfer tehnologic intre parteneri | Protocol de transfer de cunoastere dinspre IVB (CO) catre BTH (P2) privind testarea biologica in vitro a fotosensibilizatorilor pentru terapie fotodinamica. |
1 cerere de brevet depusa la OSIM | 2 cereri de brevet depuse la OSIM · Cerere de brevet A00567 din 23.09.2024 Porfirină asimetrică în matrice de hidroxipropilmetilceluloză pentru tratamentul afecțiunilor cutanate premaligne. Autori: Emma Adriana Ozon, Andreea Mihaela Burloiu, Rica Boscencu, Gina Manda, Valentina Anuta, Cristina Elena Dinu Pirvu, Dumitru Lupuliasa, Neagoe Ionela Victoria, Adina Magdalena Musuc, Mihai Anastasescu, Radu Petre Socoteanu. · Cerere de brevet A00630 din 23.10.2024 Hidrogel cu porfirina in asociere cu chitosan pentru potentiale aplicatii in dermato-oncologie. Autori: Olariu Laura, Boscencu Rica, Manda Gina, Serbu Sabina, Burloiu Andreea-Mihaela, Mihai Dragoș-Paul, Ene Manuela Diana. Brevet obtinut Derivat porfirinic pentru utilizare în teranostică. Autori: Rica Boscencu, Gina Manda, Radu Socoteanu Mihail Eugen Hinescu, Ionela Victoria Neagoe Laura Olariu, Brandusa Dumitriu. RO132752 (A0) 2018-08-30, RO132752 (B1) 2023-11-29. |
2 compusi porfirinici inregistrati la European Agency of Chemicals | Este in curs de pregatire documentatia de inscriere a 2 compusi porfirinici (P2.1 si P2.2), pentru care insa trebuie realizat un studiu toxicologic mai amplu, neprevazut in proiect. Acest livrabil, care nu a fost definitivat in proiect, este compensat de 2 publicatii ISI suplimentare. |
1 workshop demonstrativ | Workshop demonstrativ organizat la IVB (CO) in data de 8.10.2024: · Proiectul PORPHYDERM pe scurt (Gina Manda, IVB) · Modelare in silico pentru compusi porfirinici (Dragos Mihai, UMF) · Compusi porfirinici inovativi pentru terapia fotodinamica a bolilor de piele nemaligne (Rica Boscencu, UMF) · Brevet privind compusul P4.2 – Medalia de aur la E U R O I N V E N T 2024 (Laura Olariu, BTH) · Compusi porfirinici incorporati in gel pentru aplicatie topica (Sabina Serbu, BTH) · Discutii si planuri de viitor |
Rezultate suplimentare | 3 articole publicate: 1. Assessment of some unsymmetrical porphyrins as promising molecules for photodynamic therapy of cutaneous disorders. Burloiu, A.M., Manda, G., Lupuliasa, D., Socoteanu, R.P., Mihai, D.P., Neagoe, I.V., Anghelache, L.I., Surcel, M., Anastasescu, M., Olariu, L., Gîrd, C.E., Barbuceanu, S.F., Ferreira, L.F.V., Boscencu, R. Pharmaceuticals, 17(1), 62, 2024 (F.I.=4.3, Q1). This research was supported by the Romanian Ministry of Research, Innovation and Digitalization through the grant 637PED/2022 and the Nucleu grant PN 23.16.02.01/2023. 2. In silico and in vitro studies on an asymmetrical porphyrin derivative with therapeutic potential in skin disorders. Burloiu, A.M., Mihai, D.P., Manda, G., Lupuliasa, D., Neagoe, I.V., Socoteanu, R.P., Surcel, M., Anghelache, L.I., Olariu, L., Gîrd, C.E., Boscencu, R. Pharmaceuticals, 17(6), 688, 2024 (F.I.=4.3, Q1). This research was supported by the Romanian Ministry of Research, Innovation and Digitalization through the grant 637PED/2022, the Nucleu grant PN 23.16.02.01/2023, and by “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania. 3. Porphyrin photosensitizers into polysaccharide-based biopolymer hydrogels for topical Photodynamic Therapy: Physicochemical and pharmacotechnical assessments. Burloiu, A.M., Ozon, E.A., Musuc A.M., Anastasescu M., Socoteanu R.P., Atkinson, I., Culita D., Anuta, V., Popescu, I.A., Lupuliasa, D., Mihai, D.P., Gîrd, C.E., Boscencu, R. Gels, 2024, 10, 499. (F.I.=5, Q1). This research was supported by the Romanian Ministry of Research, Innovation and Digitization. |
2 lucrari comunicate: · 16th European Exhibition of Creativity and Innovation Iasi, Romania, 6-8 June 2024: Porphyrynic Derivative for Theranostics Use Patent No. 132752 B1 published in RO-BOPI, 11 from 29 November 2023, Laura Olariu, Rica Boscencu, Gina Manda, Radu Socoteanu, Mihail Eugen Hinescu, Ionela Victoria Neagoe, Brandusa Dumitriu. Comunicare poster care a primit Medalie de aur. · 23rd Romanian International Conference on Chemistry and Chemical Engineering (RICCCE), Mamaia – Constanța, Romania, 4-7 September, 2024: Morphological and spectral assessment of an unsymmetrical porphyrinic complex with biomedical potential, Elena Christen Creanga, Rica Boscencu, Roxana Trusca, Adina Magdalena Musuc, Radu Socoteanu, Mihai Anastasescu. |
Diseminare in proiectul PORPHYDERM
• Website: PORPHYDERM – National Institute of Pathology Victor Babeş – Bucharest (ivb.ro)
• 3 cereri de brevet depuse la OSIM:
- 3 cereri de brevet depuse la OSIM:
- Compus porfirinic cu potential de marker fluorescent in dermato-oncologie. Autori: Burloiu Andreea Mihaela, Manda Gina, Boscencu Rica, Neagoe Ionela Victoria, Lupuliasa Dumitru, Surcel Mihaela, Olariu Laura, Mihai Dragos Paul. Cerere de brevet nr. 202200775, publicat in RO-BOPI 5/30.05.2023.
- Porfirină asimetrică în matrice de hidroxipropilmetilceluloză pentru tratamentul afecțiunilor cutanate premaligne. Autori: Emma Adriana Ozon, Andreea Mihaela Burloiu, Rica Boscencu, Gina Manda, Valentina Anuta, Cristina Elena Dinu Pirvu, Dumitru Lupuliasa, Neagoe Ionela Victoria, Adina Magdalena Musuc, Mihai Anastasescu, Radu Petre Socoteanu. Cerere de brevet A00567 din 23.09.2024.
- Hidrogel cu porfirina in asociere cu chitosan pentru potentiale aplicatii in dermato-oncologie. Autori: Olariu Laura, Boscencu Rica, Manda Gina, Serbu Sabina, Burloiu Andreea-Mihaela, Mihai Dragoș-Paul, Ene Manuela Diana. Cerere de brevet A00630 din 23.10.2024.
- 4 articole publicate în reviste ISI, 3 in Q1 si 1 in Q2:
- Porphyrin macrocycles: general properties and theranostic potential. Boscencu, R., Radulea, N., Manda, G., Machado, I.F., Socoteanu, R.P., Lupuliasa, D., Burloiu, A.M., Mihai, D.P., Ferreira, L.F.V. Molecules, 28(3), 11492023, 2023 (F.I.=4.2, Q2). The research was supported by the Ministry of Research, Innovation and Digitalization, Romania, through PORPHYDERM project (ctr. no. 637PED/2022) and the Nucleu project PN 23.16.02.01/2022.
- Assessment of some unsymmetrical porphyrins as promising molecules for photodynamic therapy of cutaneous disorders. Burloiu, A.M., Manda, G., Lupuliasa, D., Socoteanu, R.P., Mihai, D.P., Neagoe, I.V., Anghelache, L.I., Surcel, M., Anastasescu, M., Olariu, L., Gîrd, C.E., Barbuceanu, S.F., Ferreira, L.F.V., Boscencu, R. Pharmaceuticals, 17(1), 62, 2024 (F.I.=4.3, Q1). This research was supported by the Romanian Ministry of Research, Innovation and Digitalization through the grant 637PED/2022 and the Nucleu grant PN 23.16.02.01/2023.
- In silico and in vitro studies on an asymmetrical porphyrin derivative with therapeutic potential in skin disorders. Burloiu, A.M., Mihai, D.P., Manda, G., Lupuliasa, D., Neagoe, I.V., Socoteanu, R.P., Surcel, M., Anghelache, L.I., Olariu, L., Gîrd, C.E., Boscencu, R. Pharmaceuticals, 17(6), 688, 2024 (F.I.=4.3, Q1). This research was supported by the Romanian Ministry of Research, Innovation and Digitalization through the grant 637PED/2022, the Nucleu grant PN 23.16.02.01/2023, and by “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
- Porphyrin photosensitizers into polysaccharide-based biopolymer hydrogels for topical Photodynamic Therapy: Physicochemical and pharmacotechnical assessments. Burloiu, A.M., Ozon, E.A., Musuc A.M., Anastasescu M., Socoteanu R.P., Atkinson, I., Culita D., Anuta, V., Popescu, I.A., Lupuliasa, D., Mihai, D.P., Gîrd, C.E., Boscencu, R. Gels, 2024, 10, 499. (F.I.=5, Q1). This research was supported by the Romanian Ministry of Research, Innovation and Digitization.
- Comunicari stiintifice
Prezentari orale
1 comunicare stiintifica la Conferinta Stiintifica de toamna a AOSR 2023 cu titlul ”Știința pentru o societate sănătoasă”, organizata in perioada la 21-23.09.2023 Universitatea OVIDIUS din Constanța;
Curs in cadrul Actiunii COST CA20121 „NRF2 in Noncommunicable Diseases: from Bench to Bedside”, 26-30 June 2023, Smolenice Castle, Slovacia. NRF2 in cancer and radiotherapy, Gina Manda.
Postere
- 16th European Exhibition of Creativity and Innovation Iasi, Romania, 6-8 June 2024. Porphyrynic Derivative for Theranostics Use Patent No. 132752 B1 published in RO-BOPI, 11 from 29 November 2023, Laura Olariu, Rica Boscencu, Gina Manda, Radu Socoteanu, Mihail Eugen Hinescu, Ionela Victoria Neagoe, Brandusa Dumitriu. Medalie de aur
- 23rdRomanian International Conference on Chemistry and Chemical Engineering (RICCCE), Mamaia – Constanța, Romania, 4-7 septembrie 2024. Morphological and spectral assessment of an unsymmetrical porphyrinic complex with biomedical potential, Elena Christen Creanga, Rica, Boscencu, Roxana Trusca, Adina Magdalena Musuc, Radu Socoteanu, Mihai Anastasescu.
- 3 scrisori de colaborare
Scrisoare de colaborare de la Prof. Antonio Cuadrado, Faculty of Medicine, University Autonoma of Madrid & Institute of Biomedical Research, Ciudad Universitaria de Cantoblanco, 28049 Madrid, Spain & C. de Arturo Duperier, 4, 28029 Madrid, Spain, email: antonio.cuadrado@uam.es. Domeniul de colaborare: profilul de activare a factorului de transcripție NRF2 în terapia fotodinamică și posibilitățile de modulare farmacologică a acestui factor de transcripție.
Scrisoare de colaborare de la Devrim Pesen Okvur, Izmir Institute of Technology, Gülbahçe, İzmir Yüksek Teknoloji Enstitüsü, 35433 Urla/İzmir, Turkey, email: devrimpesen@iyte.edu.tr. Domeniul de colaborare: dezvoltarea unei metode avansate, de tip lab-on-a-chip, pentru investigarea in vitro a terapiei fotodinamice.
Scrisoare de colaborare de la Prof. Prof. Luís Filipe Vieira Ferreira, Universidade de Lisboa, Centro de Química-Física Molecular, Complexo Interdisciplinar, Instituto Superior Técnico, Av. Rovisco Pais 1049-001, Lisboa, Portugal Email: LuisFilipeVF@ist.utl.pt, Tel: 351-21 84 19 252 / 246 / 039. Domeniul de colaborare: proiectarea si caracterizarea unor noi compuși porfirinici pentru terapie fotodinamică.
- Participarea in retele europene
Acțiunea COST CA20121 „Bench to Bedside transition for Pharmacological regulation of NRF2 in noncommunicable diseases”, acronim BenBedPhar, 2021-2025, Chair: Prof. Antonio Cuadrado, Universitatea Autonomă din Madrid, Spania, Vice-chair: Gina Manda (directorul proiectului PED), Grant Holder: INCD ”Victor Babeș”, România (CO al proiectului PED).
- 2 teze de doctorat în domeniul proiectului finalizate
- Nanosisteme metalice funcționalizate pentru teranostică în cancer, 2023. Doctorand Laurențiu-Iliuță Anghelache, conducător de doctorat Prof. Univ. Dr. Maria Crivineanu (USAMVB);
- Studii interdisciplinare asupra unor compuși tetrapirolici cu potențială aplicabilitate în dermatologia oncologică, 2024. Doctorand: Andreea Mihaela Burloiu, Conducător de doctorat: Prof. Dr. Dumitru Lupuliasa (UMF „Carol Davila”).
- Doctoranzi in domeniul proiectului PED
Doctorand farm. Serbu Sabina (P2): Cercetari privind obtinerea si caracterizarea unor hidrogeluri cu aplicatii in patologii ale tesutului epitelial- coordonator stiintific Prof Dr. Rica Boscencu (P1).
