Proiect PN-III-P1-1.2-PCCDI-2017-0769 (Contract 64PCCDI/2018) ONCORAD
The development of radiopharmaceuticals and techniques in nuclear oncology imaging and treatment is tailored to the molecular level
Total budget: 5287500 €
Coordinating institution: National Institute for R & D in Physics and Nuclear Engineering “Horia Hulubei” (IFIN-HH), Head of the Project: Dr. Mihai Ciubotaru
The title of the project is a component of P1: the Development of antibodies against specific markers evolutionary line myelogenous to radiodetectia, and the treatment of leukaemia myeloid, in the early stages
Budget for the project P1: 1595414 RON (Budget SET-up TALK: 423.011 RON)
In charge of the project P1: Dr. Mihai Ciubotaru
The institutions involved in the P1:
National institute for R & D in Physics and Nuclear Engineering “Horia Hulubei” (IFIN-HH);
The institute of Biochemistry of the Romanian Academy (ELBOW);
The National institute of Research, Medical and Military Cantacuzino (C);
The National institute of Research and Development in the Field of Pathology and Biomedical Sciences, “Victor Babes” (IV)
Team IV involved in the P1 Valeriu Cismasiu (in charge), Sevinci Pop, Gisela Gaina, Cristina Niculite, Stefania Rogozea/Alexandra Popa, Ioana Lambrescu, Victor Ionescu
Abstract :
The project ONCORAD and the technology of the radiobiology associated with, are built on the foundations of medicine, translationale, and the personalised medicine, and it has as main objective the development of radiopharmaceuticals to target, and techniques to the medical, nuclear, innovative diagnostic imaging and the treatment is increased in cancer. The project ONCORAD is based on the competence that is unique in the field of radioisotopes and of radiopharmaceutical agents in the Institute of accounting, (IFIN-HH), complementand with the expertise of the institutions involved with the activity of prodigious research in the biomedical field (IV, v and ELBOW), clinical (Clinical Institute Fundeni clinical institute, Bucharest, romania), as well as in the area of nanotechnology/nanomedicine (SET to the Technique of Isotopic and Molecular, Cluj-Napoca, romania). This is empowering as many aspects as possible is focused on four research projects that are interconnected, with the objectives in the field of nuclear medicine, cancer. In addition, the ONCORAD will assist in the development of the institutions of the consortium, through the employment of young researchers and their training in the fields of science and technology-leading the development of the infrastructure of the research, transfer of knowledge, technologies and good practices between all the partners of the consortium members and other interested parties. The project creates a framework for interdisciplinary collaboration between the partner institutions, thus providing the basis for the country's future participation of the consortium members in the project, the widening of the research, transfer of technology for clinical applications.
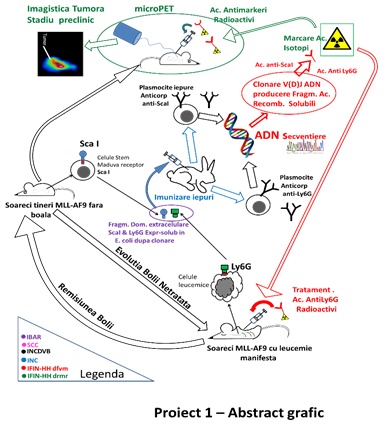
The objective of the scientific lead of the Project, 1 was getting out of the antibodies and fragments of strands coming together-Fab immune system against some of the markers, the ea of the area, both for the early stages of the stem Sca1 (stem cell antigen-1), as well as differentiating myeloid (Ly6C) present on the surface of cells of the type of mononuclear phagocytes neoplastic, in the early stages of leukaemia myeloid. These pieces, strands coming together will be labeled with stable isotopes, the emitters of electrons (Zr-89, Cu-64) in order to use them as biomarkers. The detection of the in vitro studies of the leukemic cells will be carried in the crops, techniques, nuclear power, and the in vivo micro-PET-CT (positron emission tomography coupled with computed tomography with a resolution of the sub-milimetrica). The coupling of the fragments of the immune strands coming together with the intake of therapeutic techniques are complementary, can lead to the antibody and can be therapeutic, and their effectiveness in stopping the growth of the tumor will be tested by the technique of in vitro and in vivo. Radionuclide therapy, which will be tested in the framework of the project are the emitters of radiation and transfer of energy, the high (potentially toxic) in, T-211/Ac-225 and/or With the 64/67.
The team of the IV, he developed a model of in vivo leukemia, acute myeloid, analyzed, and characterized phenotypically the cells of the tumor. Subsequently, the research group of the SET-VB it test the new anti-Sca1, anti-Ly6C, for the selection of clones of the most efficient radiolabelling.
The results of the team for the IV
In order to generate the model of acute myeloid leukemia, has been used in a line of mice, the STOCK Kmt2atm2(MLLT3)Thr/KsyJ (The Jackson Laboratory, # 009079). Transgena that mimic the translocatia cromozomala of the t(9;11)(p22;q23), is inserted into the locus of the gene Kmt2a. Translocatia lead to the synthesis of the protein of murine MLL, which is being replaced with the fusion protein MLL-AF9 (MA9). The selection of the animal carriers of the mutation was performed by genotyping by PCR. The mutation cause instability, epigenetics of hematopoietic cells, which can lead to the transformation of the oncogenica and complete the formation of the cell, leukemic stem. Therefore, the hematopoietic stem cells (HSC) and progenitor cells of the granulocyte and monocyte (G), a carrier of the mutation MA9 are considered to be cells are pre-cancerous (p,-EN,-p-G). The occurrence and progression of myeloid leukemia, has been followed by monitoring of the spleen, and the general condition of the animals. In figure 1 it is shown the curve of the survival of the animals, as well as the dimensions of the enlarged spleen (a sign of leakage of tumor cells).
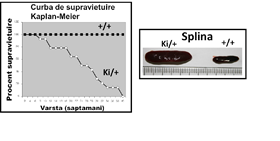
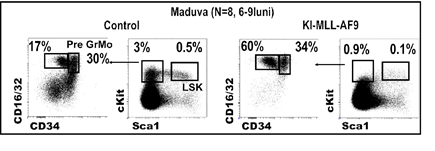
The average duration of the transforming oncogene of 25 weeks, as a result of the analysis of Kaplan-Meier. In figure 2 it can be observed that the mutation MA9 favors mielopoieza, and the growth of the populations of granulocytes and monocytes (labeled with CD45, and CD11b). The results demonstrate that the transforming oncogene, is accompanied by a decrease in the population of stem (pre -) leucemica of the p-I LOVE it (hereinafter referred LSK shown in figure 2). The second phenomenon is associated with the development of leukemia in mice MA9 is the increase in the population of precursors granulo-monocitari (pre -) with leukemia p-G (gate preGrMo in figure 2). The results are in agreement with a study that was published to the effect that the differentiation myeloid it is necessary for the transformation of the tumor and the occurrence of acute myeloid leukemia (Ye M, et al. In 2015, Cell Stem Cell 17:611-623).
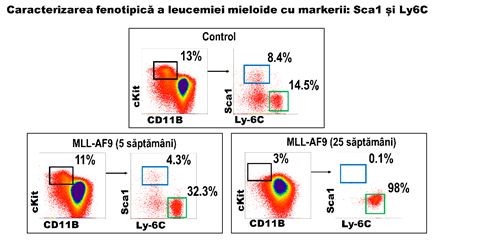
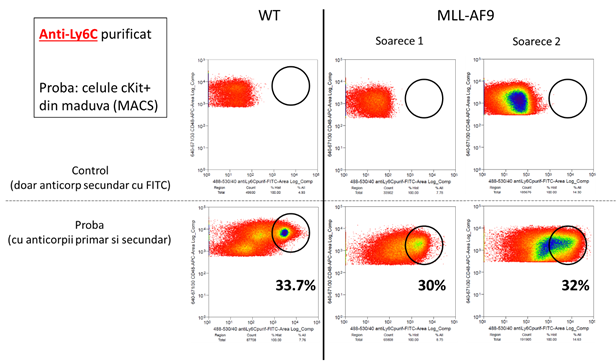
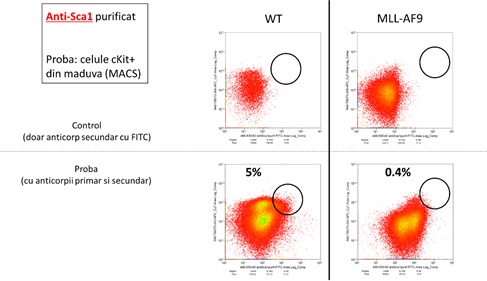
The results presented here show that the evolution of the tumor is associated with a reduction in the population of the stem and, at the same time, an increase in progenitor granulo-monocitari. Therefore, the identification of specific markers for these two types of cells will allow for the monitoring of the in vivo studies the transformation of the oncogenic. The analysis led to the identification of two markers adequate to: Sca-1, and Ly6C. In figure 3, there are shown the results are significant for a set of 4 tests independent of each other. Notice the reduction of the progenitor hematopoietici (cKit+, black rectangle) in mice MA9 at the age of 5 weeks, which means a reduction in the populations of the stem (marked with the Sca1). In line with this, we can observe a reduction of the population positive for Sca1 (figure 3, blue rectangle) for 5 weeks, and the death of these cells in mice with acute myeloid leukemia (MLLAF9, 25 weeks). In contrast, the population of the cKit-positive Ly6C-positive (see figure 3, the rectangle is green) - it keeps the number of the known as the leucemica sign of going away. Therefore, the use of the combination of these 2 markers to indicate the progression of the tumor by reducing the signal of the fluorescence generated by the anti-Sca1, and the growth of the signal of the fluorescence generated by the anti-Ly6C.
Figure 3:
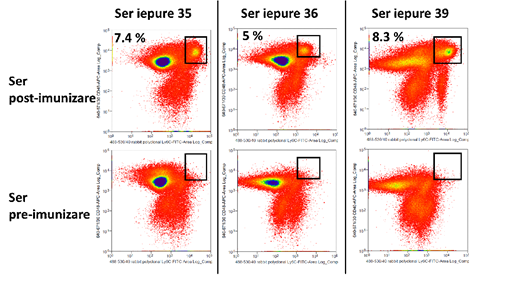
After the second round of immunization, were tested for the reactivity of the serum in the face of the blood-forming cells of mice, by the results in the stream. Immunization was done for the Ly6C, and the test samples were collected from three rabbits. The same type of cells that were stained with the antibody anti-rabbit, the marked FITC and serum (sample) - or no-serum control (background), and the diagrams, FACS, have been compared on the basis of the intensity of the fluorescent signal, and the percentage of the population is positive. In the case of the samples be treated with a serum, the binding of the antibody to the primary surface of the cell is expressed by the signal-FITC. The biological material was represented by the cells of the mouse, extracted from the bone marrow by hematogenous of the animals. The results are shown in figure 4. On the whole, the data suggest that the serum of post-immunization schedule shows the polyclonal antibodies with high affinity increased by 66% of the cases.
Figure 4:
After completing the steps to the immunization of rabbits, and the sorting of lymphocytes producing antibodies, has been in this role for a number of pieces of the immune scFV-responsive markers of antigenic Sca-1, and Ly6C. The analysis of the FACS (figure 5) indicates that all of the pieces are responsive front of the proper.
Figure 5:
In conclusion, all of the activities undertaken by the partner SET-VB has been carried out.
Publications and conferences/congresses, arising out of this topic
